By Laura Garcia-Pupo, PhD March 4, 2025
The sigma-1 protein was first thought to be an opioid receptor but was later identified as a distinct receptor chaperone protein involved in cellular stress regulation related to calcium homeostasis. Pre-clinical research has shown that the sigma-1 receptor plays a key role in neurodegenerative diseases by influencing oxidative stress and cell survival.
Blarcamesine (ANAVEX®2–73) is an oral drug candidate that targets the sigma-1 receptor and muscarinic receptors to restore cellular balance in the brain. This drug has shown the potential to protect memory and prevent brain cell damage in animal models of Alzheimer’s disease (AD) by reducing harmful protein buildup and preventing tau-related toxicity. Additionally, blarcamesine promotes the natural process of clearing cellular waste, which may help reduce inflammation and slow the progression of neurodegenerative diseases.
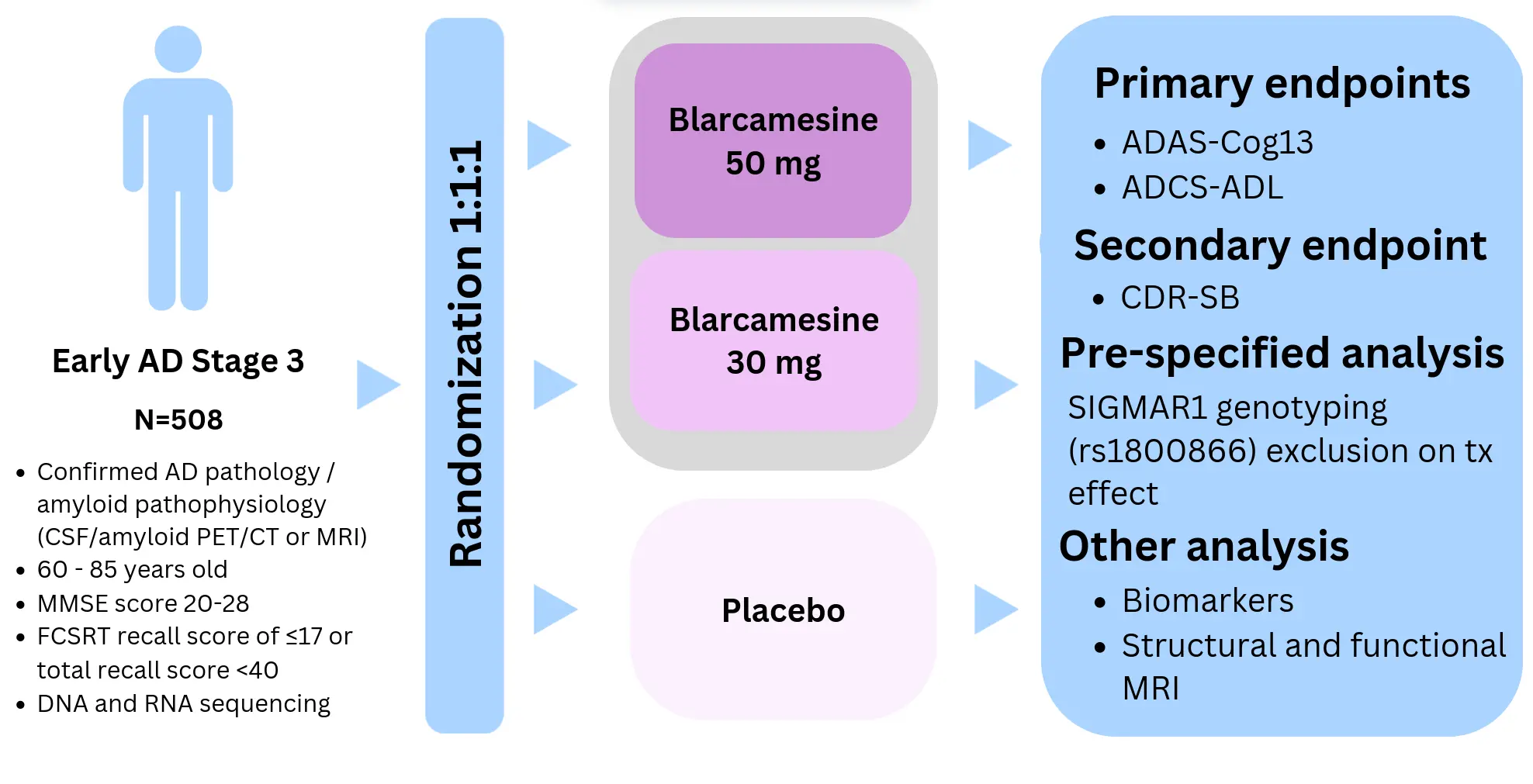
The Phase 2b/3 randomized, double-blind placebo-controlled, multicenter, international ANAVEX2-73-AD-004 trial studied the safety and efficacy of blarcamesine in patients with early AD (ClinicalTrials.gov Identifier: NCT03790709). 508 patients who met the National Institute on Aging (NIA) – Alzheimer’s Association 2011 criteria for diagnosis of mild cognitive impairment due to AD or early-stage mild dementia due to AD were randomised to oral blarcamesine (N=169 for each dose arm 50mg or 30mg) or placebo (N=170).
ANAVEX2-73-AD-004 study design
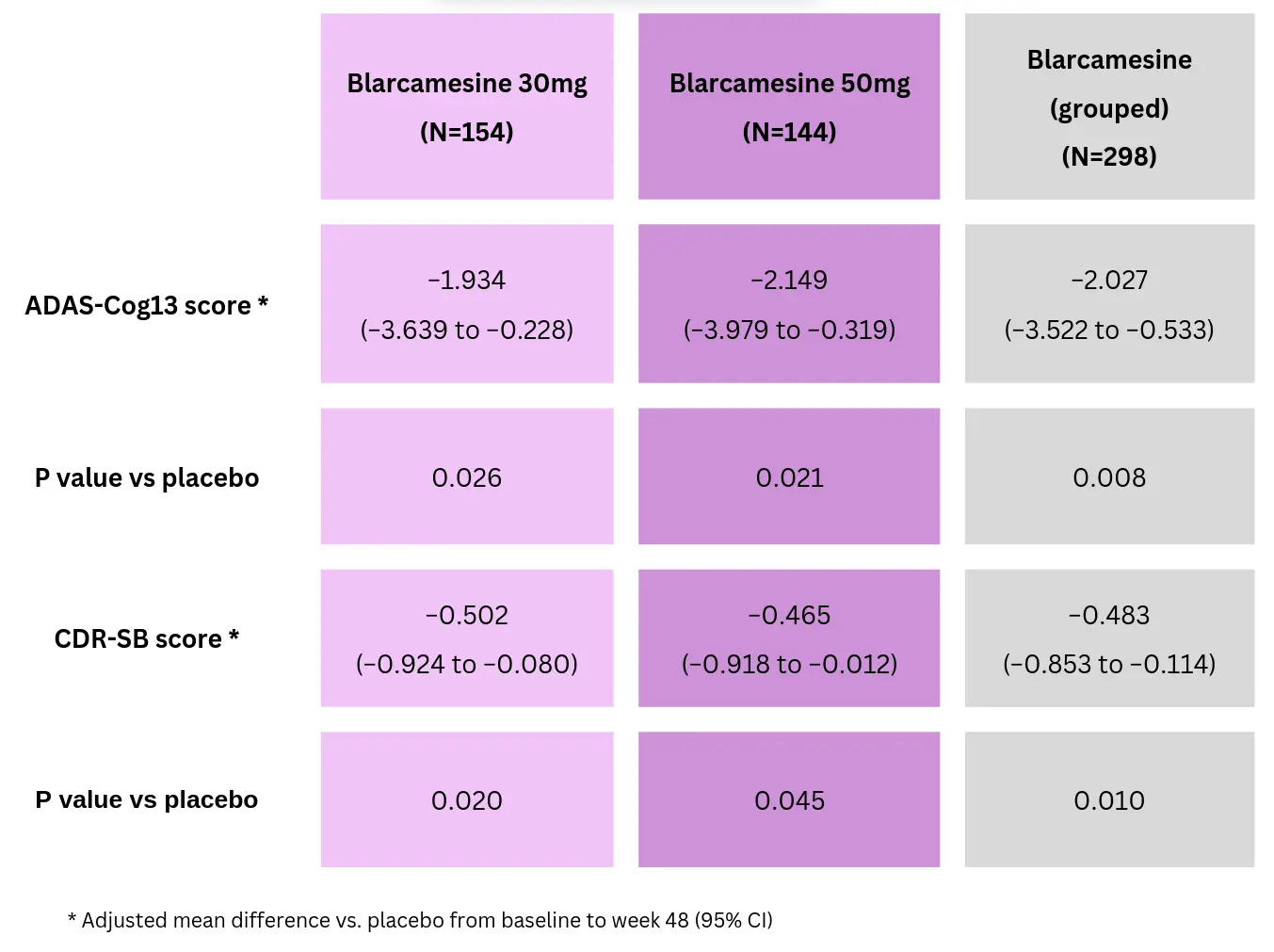
Among 462 randomised patients in the Intention-to-treat population, 338 (73%) completed the study. At Week 48, blarcamesine significantly improved the primary endpoint ADAS-Cog13, representing a 36% and 39% reduction in clinical decline in all patients receiving blarcamesine (grouped) and those receiving 50mg of blarcamesine, respectively (see table below). However, the co-primary endpoint ADCS-ADL did not reach statistical significance at 48 weeks. Blarcamesine significantly reduced the decline in the secondary endpoint CDR-SB vs placebo in all patients receiving the drug (28%), and those treated with 30mg (29%) or 50mg (27%).
Improved cognition and function outcomes by blarcamesine vs placebo
Among all the blood biomarkers evaluated, plasma Aβ42/40 ratio was the only one increasing significantly in all patients receiving blarcamesine (mean difference vs. placebo=+0.013; 95% CI: 0.000-0.026; P=0.048) from baseline to week 48. However, the changes in plasma Aβ42/40 ratio among patients treated with either 30mg or 50mg of blarcamesine did not reach statistical significance vs placebo at week 48 (Supplementary Table 3).
Brain imaging showed a significant reduction of the total grey matter volume loss at week 48 from baseline in all patients receiving blarcamesine (adjusted mean difference vs placebo=1.4%; 95% CI: 0.5-2.3; P=0.004), and in patients treated either with 30mg (adjusted mean difference vs placebo=1.6%; 95% CI: 0.5-2.6; P=0.003) or 50mg (adjusted mean difference vs placebo=1.1%; 95% CI: 0.0-2.3; P=0.049). The enlargement of lateral ventricles also significantly decreased in all patients receiving blarcamesine, as well as in the individual treatment arms (Supplementary Table 4).
Grade 1 or 2 dizziness and confusional state were the most common adverse events (5% or more) during treatment titration and maintenance. Treatment-emergent adverse events leading to study discontinuation occurred in 32% of blarcamesine and 7% placebo groups, mainly before week 12.
The ATTENTION-AD trial (ClinicalTrials.gov Identifier: NCT04314934 is an open-label extension study to evaluate the safety and efficacy of oral once daily blarcamesine in participants with early AD at a longer term. The delayed-start analysis of cognition and function co-primary endpoints showed more favorable outcomes in the early-start treatment compared with the late-start treatment. The full data will be presented at the AD/PD meeting in April, 2025.